Categories
Speciality Areas
Innovation – How Do We Get ‘Better’?
Innovation Comes in Many Guises – How Can We Make the Most of Them?
Under the heading of innovation in healthcare we see the process of developing and adopting new or improved therapies, technologies, products, services, and processes, to improve patient outcomes, increase access to care, and enhance overall healthcare system performance. Innovation encompasses everything from major breakthroughs like new drugs and treatments to smaller, incremental, changes like redesigned workflows or digital tools, with the ultimate goal of meeting personal and public healthcare needs more effectively and efficiently.
There can be considered to be four types of innovation – disruptive (e.g. AirBnB, Uber, film or music streaming), incremental (e.g. camera enhancements from one version of an iPhone to the next), sustaining (e.g. each new model of a car has a new benefit to keep it ahead of the competition), and radical (e.g. cell phones). In healthcare, each type offers distinct pathways to solving market challenges and each has a contribution to make to healthcare.
In Assistive Technology, the majority of innovations are incremental, and most of the examples covered in this article fall into this category. For example, this might entail more sophisticated electronics on a powerchair that give the occupant a smoother ride or more access to a wider selection of devices. Many of the innovations ‘on show’ at exhibitions like Naidex, Posture & Mobility Group, Rehacare, etc, will not be obvious to the naked eye, and so a quick glance and walk by will mean that you’ve missed the hidden gem. So do take the time to ask what’s new, and what are the advantages and benefits of each innovation.
Drivers for Innovation
There are various drivers behind the innovations we have enjoyed in the realm of Assistive Products. Some of these have arrived on the back of new legislation, new product ISO, EN or BS standards, or even geo-political changes. Some have come from the advantages that modern electronics, or new materials can offer. Some from a creative or lateral-thinking approach to solving a clinical challenge, or directly from user experience. There are also commercial drivers to keep ahead of the competition, and to keep products up-to-date, both physically and aesthetically, in the eye of a product’s user.
Material Differences

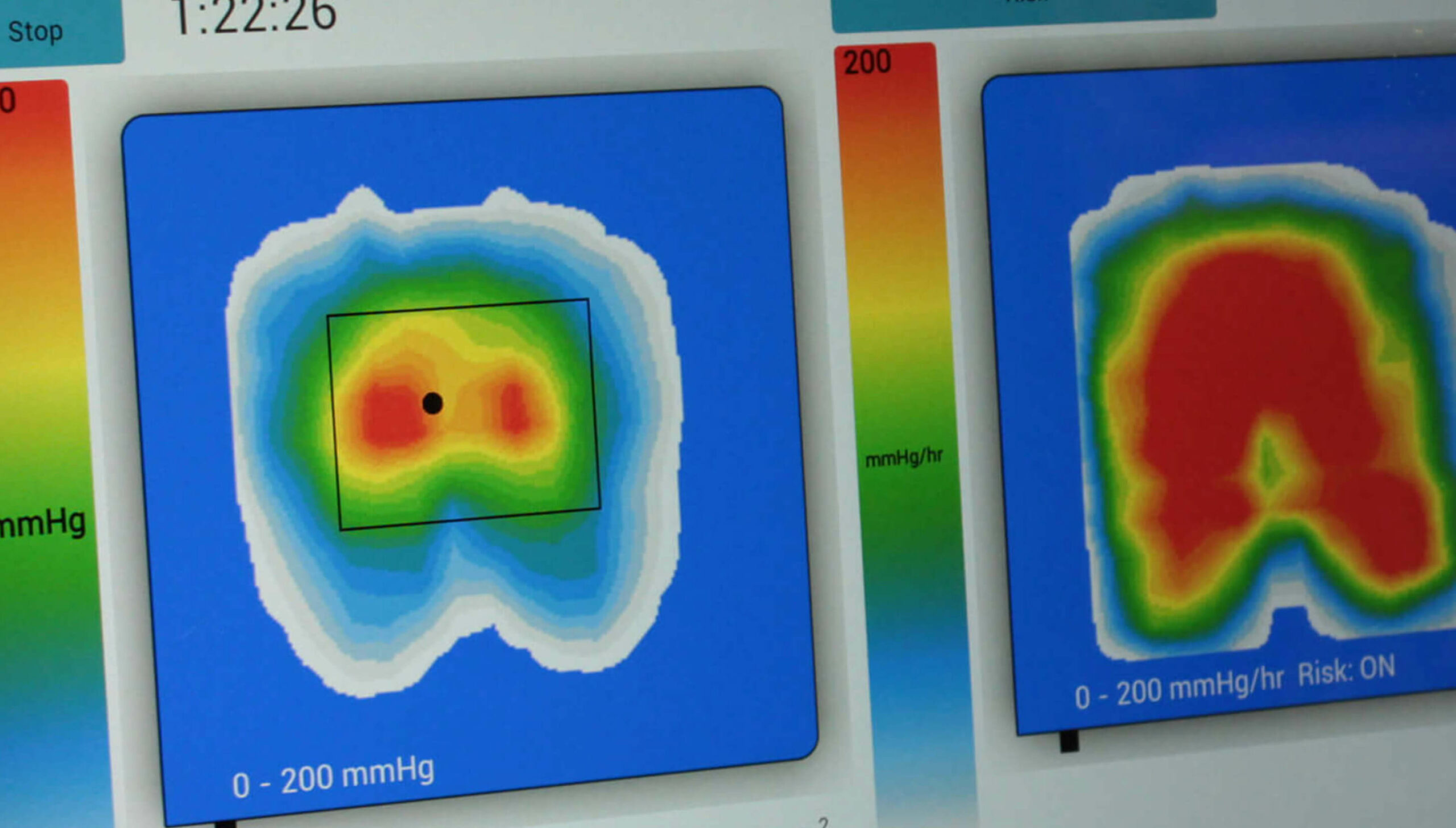
For assessing individuals’ postures and risks of skin tissue injury, I have seen over the years the radical innovations in pressure-mapping technologies with the progress from a ‘clinic-based’ small array of expensive and fragile individual sensors that took a one-off reading from each sensor, to portable systems with materials, technology and software that give continuous arrays of data very quickly – and at a price that is affordable for nurses in the community to be able to take out with them routinely, and that are so simple to use that the fear of new-technology factor has been taken away.
On the geopolitical front, the NHS has been introducing increasingly strict requirements on suppliers relating to carbon footprints and zero-carbon policies. This initiative has been concentrating on manufacturers and manufacturing processes. There is hope that they may take this through into the actual products procured, with more emphasis on a circular economy 1.
A good candidate in the latter category has been the introduction of 3D spun thermoplastic elastomers – of the type to be found in the NEST pressure care products (Figure 2).

For skin health, the NEST can provide comparable pressure care to a foam cushion or mattress, but also the 3D random loop structure of the polymer web absorbs various of the shear strains under the skin tissues. The open structure allows for heat and water-vapour dissipation. The structure is maintained in use and provides a ‘springiness’ which facilitates movement, enabling turning in bed or movement on a cushion, thereby offering further pressure-redistribution properties and aiding independence. From the carers’ point of view, the light weight means that NEST mattresses can be lifted and turned by a single person, providing a major manual-handling advantage. Unlike polyurethane foam, the NEST core material can be washed and disinfected whenever necessary, and also unlike foam, the material can be chopped up and melted down to be reused in new product – also avoiding the need of disposal in landfill or incineration, which are both expensive and damaging to the environment.
Radical Changes
In recent years we have seen some ‘radical’ changes in the wheelchair-seating world. One example of where modern electronics can make a large difference was the introduction of the iDrive by Stealth (Figure 3), wherein a person who cannot use a joystick to drive a powerchair, but could use alternative controls, can now drive their chair just as smoothly, thanks to the speed and algorithms incorporated into the software.


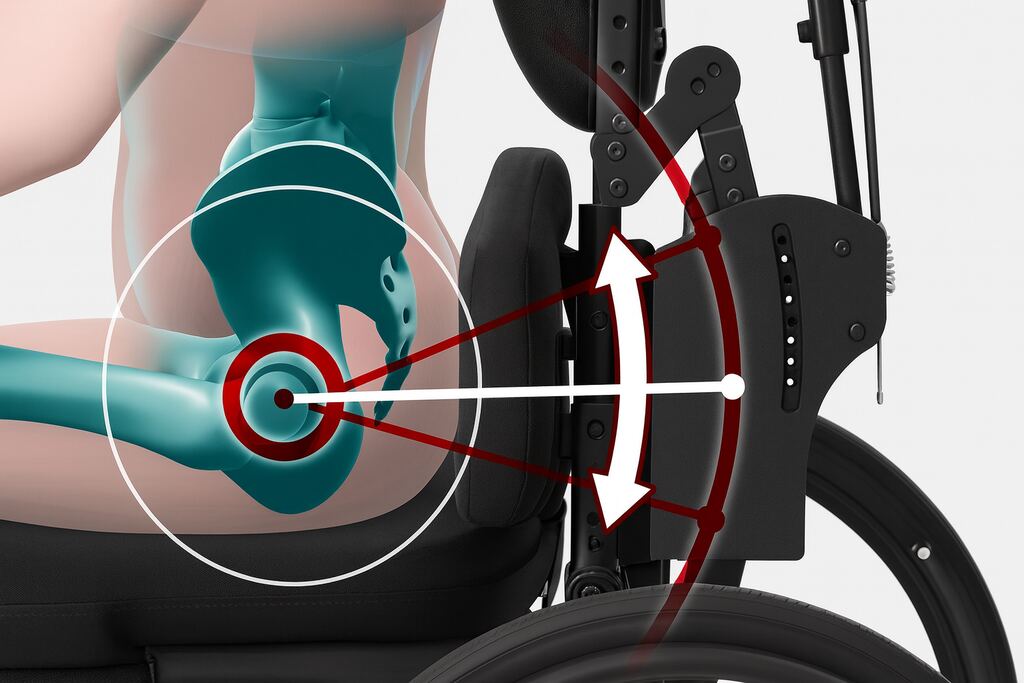
Other radical changes have come from products which match the person’s biomechanical function. One example is Bodypoint’s Dynamic Arm Support (Figure 4), which supports the arm in an anatomically correct position at the shoulder joint and forearm, enabling improved function and reducing the risk of pain and shoulder subluxation for the user (in contrast with what an ‘arm rest’ on a standard chair does).
Another example is the EpiC seating system (Figure 5), again from Stealth, which facilitates the back support to move in parallel with changes of angle at the hip joint, removing shear forces from the back and keeping the accessories around the torso and head in constant alignment with the trunk as the upper body moves.

Incremental Changes
The Tripp Trapp children’s chair is a feature in many homes around the world. It was originally designed in 1972 by Peter Opsvik, for his son Tor, and millions have been sold since. A Tripp Trapp is on the ‘wall of fame’ in the Design Museum in London displaying what people have voted as design icons.
Around 40 years ago, Jon Prout in the UK saw the benefits of the chair’s ability to grow with a child, but found that the basic design had shortcomings for use with children with behavioural and physical challenges, and so he created the Breezi™, which had an extended footprint at the front to reduce the risk of tipping over, and accessories to accommodate the more special needs of the challenged youngsters. He broadened the range as well, into three sizes to accommodate the needs of different markets. The innovation was to adapt something that existed for a general audience to make it accessible to a wider market. Other manufacturers have designed their own variations on a height-adjustable chair, but the Breezi is the only one with the DNA of a Tripp Trapp.

Nearly ten years ago Jon’s company was merged into BES Healthcare in Bristol, and BES has been working on incremental changes to meet the subtle changes that have evolved in the Breezi’s marketplace. One example was the early modification of the slots in the seat plates for placement of the pelvic positioning belts to meet initially BS 8625 and latterly ISO/TS 16840-15, where the belts needed to be able to be placed across the thighs and in front of the greater trochanters – an example where new standards have driven change.
The knock-on from this was the child’s pelvic belt was now positioning them properly and they did not need a fixed pommel to stop them sliding out of their seat, and instead the quick-release alternative could be used appropriately as a medial knee support. The positioning benefit was supported further by the introduction of an optional pre-ischial pad under the seat pad, which also helped to reduce the tendency of the child to slide forward.
Some of the incremental changes have been more subtle. Slight variations in the back-rail dimensions between the larger and smaller versions meant that it was difficult to put a wider-width seating system into the medium-height version. These have all been standardised so that customers can have full interchangeable modularity between the original three heights and the three widths, and that if, for example, over time the height is still right but the width is now too small, they can just upgrade the width-related items.
Sustaining Changes
Earlier this year, BES introduced a smaller-height model to the Breezi range, in the form of the Teeni (Figure 7). This ‘innovation’ fits in the ‘sustaining’ category. This model uses the same components as the other versions in the Breezi range, and it’s only the ‘z-legs’ which differ. The height has been designed for a nursery or other environments with toddler-height tables. For greater flexibility the foot plates can be mounted at standard incremental heights from the floor upwards.

Resistance to Innovation
‘Innovation’ implies change. Change can take us out of our comfort zones. But, apart from taking people out of their comfort zones, why is it so difficult to get any innovation taken up within the NHS?
There is the adage that “no-one has ever been sacked within the NHS for doing what has always been done”. This inertia has also been exacerbated by juniors learning at the feet of their seniors, and so local and older ways are passed on. However, if the senior has invented a new way of doing something, that will be included. Which leads us to the ever-present NIH (Not Invented Here) syndrome. There have been many pilot studies carried out in individual Trusts or communities, with great clinical or financial benefits established, but these seldom lead to adoption elsewhere. Even a positive NICE report does not often lead to broadened take-up.


A change in regulatory aspects will often be overlooked if the institution thinks there is no oversight and they can get away with it, with the driver being the avoidance for as long as possible in incurring an additional cost to meet the legislation. An example of this is that it is implicit in the MDD, and explicit in the EU MDR, that reusable medical devices must be processed (washed and disinfected) by or within devices that are themselves registered as medical devices. As a minimum a washer has to be a Class I device, and a washer-disinfector or disinfectant a Class IIa device. But how many bodies that are reprocessing medical devices are actually currently compliant?
Procurement Inhibitors
Another uptake inhibitor is that procurement policies are often restrictive and fail the system by only allowing the tendering of existing equipment and technologies, with the only guideline, in reality, being how low a price can be achieved on day one. It’s easiest just to buy the same items as we bought before, and it’s easiest to write a tender around products rather than the outcomes or benefits that a tenderer’s offered products might provide.
There tends to be nothing built into tender scoring around incorporating any elements of a circular economy. There is little regard as to what a little extra cost today might have on long-term savings in both costs or patient well-being. Sometimes the inherent silo-thinking around “why should I spend a bit more in my department when it only saves another department some costs”, blocks the adoption of innovation, or even cost-effective procurement.
Conclusion
In conclusion then, it’s inertia within the colossus that is our health and social provision structures that will continue to block innovation. Until government starts to lead from the top and directs local procurement to change their ways: by doing what we have always done, we will get what we’ve always got!