Categories
Speciality Areas
Sound bases for clinical practice and procurement

Where do we get the assurance that our assessment and prescription practices are following best practice? Often this comes by word of mouth, but sometimes the words can get corrupted by the process of ‘Chinese Whispers’.
Procurement departments frequently rely on clinicians to advise them as to what to order, while clinicians often hope their procurement departments have the training to source appropriate products. The end result can be the blind leading the blind, and the patient not necessarily getting the best solution – and in many cases the lowest price option is not the best long term solution. This article addresses one direction to which one can turn for sound advice.
Most individuals involved in prescribing assistive products will probably have been through a relevant college or university course, though how ‘relevant’ this teaching is to many real life situations is a key question. An occupational therapist, for example, will have finished their course with very little related to wheelchair prescription or the principles behind sound seating assessment having been covered. What does a product need to be approved to be considered to be an appropriate medical device, and why is this important? Where does one go for advice on best practice, for advice on what and where to ask the right questions concerning the different clinical properties of possible solutions, or for advice on what is safe for the client and also safe for the carer?
Medical device?
A product is a medical device if it has a medical CE mark and/or, since the UK left the EU, a medical UKCA mark. To have this mark, the product needs the manufacturer to have stated what claims of patient benefit they are making for the product, and then a supporting technical file providing the evidence for these claims. The nature of the claims will lead to the product classification. Class I products will be self-certified by the manufacturer, but if a higher class, this means they will have had to have the documentation verified by an external body. To justify their claims, the manufacturer will have carried out clinical trials, if feasible, or otherwise tested their product against appropriate standards or methodology.
Where do standards come in?
International, and European, standards variously provide internationally agreed means for safety testing, and are therefore a good starting point for a CE/UKCA technical file. In addition, there are also international performance standards which can be used to support the clinical claims made by the manufacturer. For general requirements for Assistive Products visit ISO 218561, and for wheelchairs EN 121832 and 121843 as starting points. But standards do not just stop there – they can also offer internationally agreed approaches to best practice. In the following part of this article, examples of the different types of standard for these criteria have been selected from those for seating systems.
Safety
A first element of safety for any assistive product is that it will not catch light if near a heat source, or that any fire self-extinguishes quickly. ISO 16840-104 specifies a surrogate cigarette test (see Figure 1) for this, and has been described in more detail in a previous THIIS article5.
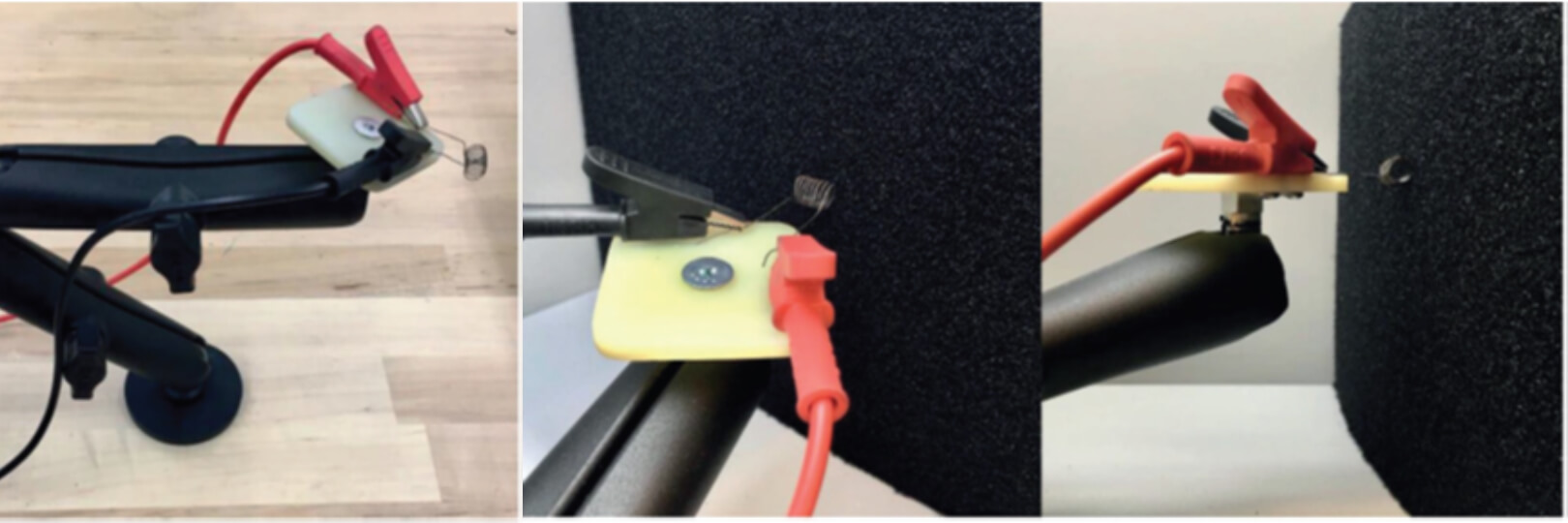
For any wheelchair user who also uses positioning belts and harnesses, it is important that the webbing and buckles will stand up to continued use and forces, and that they do not move or slip when in position. ISO 16840-36 covers pass/ fail criteria that these and other postural support devices should be tested to. Figure 2 shows an example of a failure of a pelvic belt tested to this standard.

For any wheelchair user who also uses positioning belts and harnesses, it is important that the webbing and buckles will stand up to continued use and forces, and that they do not move or slip when in position. ISO 16840-36 covers pass/ fail criteria that these and other postural support devices should be tested to. Figure 2 shows an example of a failure of a pelvic belt tested to this standard.
Performance
How do you know that one cushion is better for your client than another? There are up to 30 criteria by which the performance of one cushion can be compared with another. Some of these criteria relate to tissue integrity, some to postural management, and some to aspects of functionality. These are not pass/fail criteria, but measures of performance. An example of differential requirements might be that one client wants a cushion with a slippery cover so that they can slide off it easily, while another client wants one that stops their sliding about. So clearly one cushion will suit that one client while another will be better for another client. This is why it is so important that procurement exercises are conducted around clinical outcomes, and not around what a product is made from. A previous THIIS article8 looks at cushion performance testing in more detail, and includes performance testing from the likes of ISO 16840-29, and the changes with accelerated aging of these characteristics, as covered by ISO 16840-610 .
Best practice
However, international standards do not just cover testing methodology. They can also address terminology and best practice. The first of the seating standards, ISO 16840-111 , described measures of the body and of seating systems, and from this a consistent way of measuring, say, the degree of a pelvic obliquity (see Figure 3), and its changes with time. The technical aspects have been converted into a Clinical ApplicationGuide12 which gives advice about how to take the measures described in the standard.
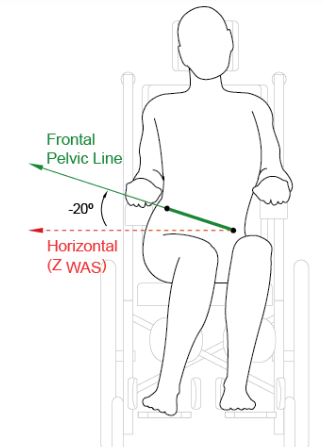
Figure 3. Figure 2.25 Frontal pelvic angle from the Clinical Application Guide.
Other guidelines include one on pressure mapping (ISO 16840-913 – the key content of this can also be found on the PMG Best Practice website14. There is a useful summary document (ISO/TR 16840-1415) which covers the differences between pressure, shear, and friction. The most recent guideline publication is ISO/TS 16840-1516 (which replaces the British version BS 8625) which gives guidance on the selection, placement, and fixation of postural support devices.
A further good resource for advice on best practice can be found on the RESNA website17 in the US.
What else?
ISO wheelchair standards work is in process around developing the same degree of coverage of back supports as there is for cushions. In the meantime, the committee is also working on new documents covering assessments, with one on the value of a seating assessment, including a listing of a minimum dataset and categories to be measured, and another on how to translate Range of Motion assessments into linear seating measures. Measurement of shear forces and assessing their effects is another area of development, including where computer modelling and simulation can provide us with data where two-dimensional physical measures are not feasible or relevant. AI is now coming into the wheelchair seating assessment arena, with research in Japan showing AI generated data providing means to obtain relevant measures of parts of the body where it is difficult to get to for normal physical measures.
What can we take from this? Newcomers to the arenas of wheelchair and seating prescription have a very specialist furrow to plough. It’s a three-dimensional world and where sitting in itself is an activity. With each client having each such individual needs, it takes years of hands-on experience to gain the depth of knowledge to do the job instinctively. But there are some valuable starting points that will lead to better practice, and more appropriate solutions for your clients. Unfortunately, today’s lower cost solution may well lead to more costly interventions tomorrow! Procurement processes should be looking at both immediate and also long term outcome benefits and total lifetime costs.
References
A guide to all the wheelchair standards can be found on the ISO TC173 web library:
- ISO 21856:2022 Assistive products – General requirements and test methods
- EN 12183:2022 Manual wheelchairs – Requirements and test methods
- EN 12184:2022 Electrically powered wheelchairs, scooters and their chargers – Requirements and test methods
- ISO 16840-10:2021 + Amd1:2024 Wheelchair seating — Part 10: Resistance to ignition of postural support devices – Requirements and test method
- Medical Device Flammability Testing THIIS April 2024
- ISO 16840-3:2022 Wheelchair seating — Part 3: Determination of static, impact and repetitive load strengths for postural support devices
- ISO 16840-4:2009 Wheelchair seating — Part 4: Seating systems for use in motor vehicles
- What make a good cushion? 7. Standards of evidence THIIS June 2022
- ISO 16840-2:2018 + Amd1:2024 Wheelchair seating — Part 2: Determination of physical and mechanical characteristics of devices intended to manage tissue integrity – Seat cushions
- ISO 16840-6:2015 Wheelchair seating — Part 6: Simulated use and determination of the changes in properties — Seat cushions
- ISO 16840-1:2006 Wheelchair seating — Part 1: Vocabulary, reference axis convention and measures for body segments, posture and postural support surfaces (under extensive revision)
- Waugh, K and Crane, B (2013) A Clinical Application Guide to Standardised Wheelchair Seating Measures of the Body and Seating Support Surfaces https://www.pmguk.co.uk/ resources/best-practice-guidelines/bpg-archive
- ISO/TR 16840-9:2015 Wheelchair seating — Part 9: Clinical interface pressure mapping guidelines for seating
- BPG 2 Clinical guidelines for the use of interface pressure mapping for seating https://www.pmguk.co.uk/resources/ best-practice-guidelines/bpg-archive
- ISO/TR 16840-14:2023 Wheelchair seating — Part 14: Terminology and concepts related to managing external forces to maintain tissue integrity
- ISO/TS 16840-15:2024 Wheelchair seating — Part 15: Selection, placement and fixation of flexible postural support devices in seating
- https://www.resna.org/